Antimicrobial Resistance Explained: Causes and the Role of Diagnostics
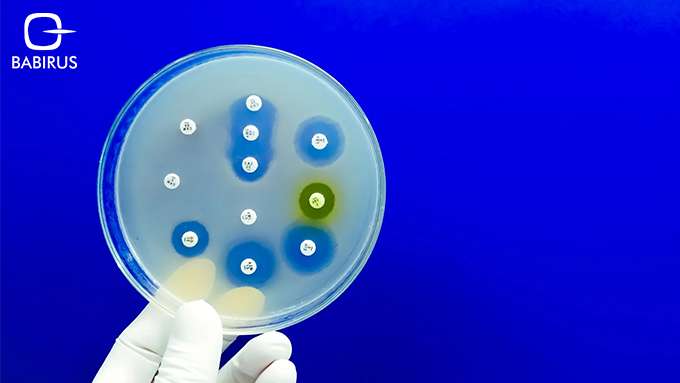
Antibiotics were once celebrated as life-saving breakthroughs that transformed the way infections were treated. Today, the very tools that once turned fatal illnesses into manageable ones are under pressure. Bacteria, fungi, and other microbes are adapting, evolving, and in many cases, pushing past the limits of modern medicine. This growing challenge is known as antimicrobial resistance (AMR).
You may have heard the term “superbugs”, microorganisms that no longer respond to medications that once worked. These aren’t just science fiction villains; they’re very real and spreading in hospitals, communities, and even the food supply. What makes this problem worse is the lack of accurate infection diagnosis in many settings, leading to inappropriate antibiotic use and poor treatment outcomes.
Continue reading to learn what antimicrobial resistance really means, what causes it, how it spreads, and the role of diagnostics in identifying and managing resistant infections.
What Are Antimicrobials?
Before diving into resistance, it’s important to first understand what antimicrobials are. These are medications designed to treat infections caused by various microorganisms. They target bacteria, viruses, fungi, and parasites, each category requiring a different class of drugs. The main types of antimicrobials include:
- Antibiotics – used for bacterial infections.
- Antifungals – prescribed to treat fungal conditions like candidiasis.
- Antivirals – taken to control viruses such as influenza, HIV, or hepatitis.
- Antiparasitics – given for parasitic infections like malaria or giardiasis.
Each antimicrobial works by either stopping the microbe from multiplying or by killing it outright. This could involve blocking a critical process the pathogen needs to survive, like protein synthesis or cell wall construction. When they work as intended, these medications reduce the spread of infection and help the body recover.
But microbes don’t stay the same. Over time, they adapt to these drugs in ways that help them survive, rendering our go-to treatments less effective. This adaptive process is known as antimicrobial resistance, and it’s rapidly changing infection management worldwide.
What Is Antimicrobial Resistance?
Antimicrobial resistance occurs when bacteria, viruses, fungi, or parasites change over time and become resistant to the medicines designed to eliminate them. These changes don’t happen overnight, they’re often the result of decades of misuse, overuse, and lack of proper diagnosis.
At the center of AMR are bacteria that become resistant to antibiotics, making even minor infections harder to treat. But AMR isn’t just about bacteria. Resistance can develop in parasites (such as those that cause malaria), fungi (like Candida auris), and even some viruses. And when medicines no longer work, treatments become more complicated, expensive, and sometimes even unavailable.
What Are the Types of Antimicrobial Resistance?
Antimicrobial resistance can be grouped into two categories: intrinsic resistance and acquired (or extrinsic) resistance.
Intrinsic Resistance
Intrinsic resistance occurs when a microorganism naturally has features that protect it from the effects of a specific antimicrobial. This might be due to its outer structure or how it functions internally. For example, certain bacteria may have a cell wall that naturally prevents specific antibiotics from entering, making those drugs ineffective from the start.
Acquired (extrinsic) Resistance
Acquired resistance, on the other hand, develops over time. A microbe that was once sensitive to treatment may undergo changes, either through mutations or by acquiring resistance genes from other microbes, that allow it to survive exposure to the drug. These changes can then be passed along as the microbes reproduce or even shared directly between organisms, especially among bacteria.
Examples of antimicrobial resistance include:
- Bacteria like MRSA or carbapenem-resistant Enterobacterales (CRE), which no longer respond to commonly used antibiotics.
- Fungi such as Candida auris and Aspergillus fumigatus, which have shown resistance to antifungal medications.
- Viruses like HIV, hepatitis C, or influenza, which may develop resistance to antiviral treatments over time.
- Parasites, including those that cause malaria or leishmaniasis, which in some cases no longer respond to drugs that once worked well.
It’s worth noting that not every infection from these organisms is resistant, many cases still respond to treatment. But growing resistance means that routine care is becoming more complicated, and accurate testing is more and more important.
What Causes Antimicrobial Resistance?
The rise of antimicrobial resistance is linked to several key drivers:
Overuse and Misuse of Antibiotics
Antibiotics are still widely prescribed for infections that are viral, not bacterial. Since antibiotics don’t work against viruses, their use in such cases offers no benefit and contributes to resistance. Similarly, stopping a course of antibiotics too early allows bacteria to survive and develop resistance.
Lack of Rapid Infection Testing
Many healthcare settings still rely on outdated or slow diagnostic methods. Without tools that can quickly distinguish between bacterial and viral infections, antibiotics are often prescribed as a precaution. This guesswork creates unnecessary pressure on antimicrobial drugs.
Hospital-Acquired Infections
Hospitals and other clinical environments are hotspots for resistant pathogens. When patients stay for extended periods, are immunocompromised, or undergo frequent treatments, the risk of developing resistant infections increases. If these infections aren’t identified quickly, they can spread to others and complicate recovery.
Agricultural Antibiotic Use
Antibiotics are commonly used in livestock and poultry to promote growth or prevent illness. This contributes to the emergence of resistant strains that can be passed to humans through food, water, and direct animal contact.
The Role of Diagnostics in the Fight Against AMR
One of the most effective ways to slow antimicrobial resistance is to use diagnostics to guide treatment. When healthcare providers know the cause of an infection, and which medications will or won’t work, they can treat patients more accurately and avoid unnecessary antibiotic use.
Here’s how modern diagnostics are changing the fight against AMR:
Molecular Testing for Resistant Bacteria
Polymerase Chain Reaction (PCR) is a highly accurate method that detects genetic material from microbes, including resistance genes. PCR-based diagnostics can identify resistant pathogens in a matter of hours, unlike traditional culture methods that can take days.
Multiplex PCR for Bloodstream Infections
Sepsis is a major cause of hospital mortality, often made worse by drug-resistant bacteria. Multiplex PCR tests can identify multiple pathogens and resistance markers in a single sample, helping providers start the right treatment faster.
Point-of-Care Diagnostic Tests
In outpatient and emergency care settings, fast testing can be the difference between overprescribing and targeted therapy. New point-of-care tools like FebriDx are helping clinicians make informed decisions right at the bedside. This single-use test detects bacterial versus viral infections in under 15 minutes, offering a clearer direction on whether antibiotics are warranted. Instead of depending on broad-spectrum treatments, healthcare providers can deliver more focused care, helping to reduce unnecessary antibiotic use and support better infection control practices.
Antimicrobial Stewardship with Better Testing
The more accurate the test, the more responsible the treatment. Avoiding unnecessary prescriptions helps diagnostics play a key role in conserving the effectiveness of antibiotics. This forms the backbone of antimicrobial stewardship, a practice that promotes thoughtful antibiotic use to reduce resistance and improve outcomes.
The Future of AMR Control
To keep pace with evolving resistance, healthcare systems must look ahead. Here’s where AMR control is headed:
Smart Testing Technologies
Next-generation diagnostic tools are focusing on speed, accuracy, and accessibility. Portable devices capable of processing results within minutes are in development, aiming to make diagnostics more efficient even in rural or underserved areas.
Broader Surveillance and Global Data Sharing
Linking diagnostic data with global health monitoring systems allows public health organizations to track resistance patterns more effectively. Early alerts allow for quicker action during outbreaks and better resource planning.
Expanded Use of Multiplex Panels
Multiplex testing will become more common, especially during outbreaks. With the ability to test for multiple bacteria and resistance genes in one go, it offers a streamlined and cost-effective alternative to traditional methods.
Companion Diagnostics for New Drugs
As new antibiotics and antimicrobials are developed, companion diagnostics will help identify which patients will benefit from which treatments. This targeted approach can reduce resistance and improve success rates.
Lastly,
Antimicrobial resistance is reshaping the way infections are managed, and not in our favor. But it isn’t a challenge we’re powerless against. Smarter diagnostics are already proving they can change outcomes by delivering faster answers and guiding better decisions at the point of care.
Whether it’s pinpointing resistance genes through molecular tools, distinguishing between bacterial and viral infections with rapid tests like FebriDx, or using multiplex panels to tackle bloodstream infections, diagnostics are shaping a more informed, more focused approach to treatment.
At Babirus, we support that shift. With innovative solutions designed to help labs act faster and with greater confidence, we’re committed to strengthening the fight against resistance; one accurate result at a time.